Subsurface hydrochemical precision treatment of a coastal acid sulfate soil
This publication was published in Applied Geochemistry, Volume 100, January 2019, Pages 352-362. Link to publication: https://www.sciencedirect.com/science/article/abs/pii/S0883292718303548?via%3Dihub and here: https://research.abo.fi/converis/portal/Publication/16313704
Abstract
Some of the most economically valued soils for agricultural use are naturally occurring sulfide rich sediments. However, formation of acid sulfate soils with sulfuric materials (pH ≤ 4) can occur when sulfidic materials are exposed to air, which can then result in mobilisation of large amounts of acid and metals into nearby water bodies. In this study, controlled drainage, subsurface irrigation and hydrochemical precision treatments are combined to reduce acidic discharges on a novel project field in western Finland.
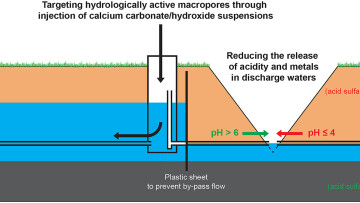
The PRECIKEM project field consists of nine identical hydrologically isolated 1 ha subfields. Each field had a drainage system consisting of three subsurface drainage pipes (c. 1.3 m deep), a collector pipe, and a control well enabling manual groundwater table management. Utilising such drainage installations already common on farmlands, suspensions of fine-grained (d50 = 2.5 μm) calcium carbonate and/or calcium hydroxide were pumped in to control wells in order to be distributed in to subsoils with sulfuric materials via drainage networks with the aim to: (1) neutralise acidity, (2) inhibit microbially mediated sulfide oxidation and (3) immobilise metals. The discharge waters from the fields were monitored during the project period 2012–2016. As is typical for acid sulfate soils with sulfuric materials, the discharge waters from the reference fields (n = 3) that had been treated with water only, had very low pH values (≤4) and the acidity and concentrations of several metals were up to two magnitudes higher than the average in Finnish stream waters.
Excavation of selected treated fields revealed the calcium carbonate to have formed a neutralising coating on the surfaces of hydrologically active macropores in the soil matrix near the subsurface drainage pipes. This effectively resulted in a long-term (1–4 years) situation of raised pH, lower acidity and lower concentrations of several acid sensitive metals, most prominently a significant decrease (>90%) in Al concentrations. Fe concentrations in discharge waters were subsequently decreased as the predominance of Fe shifted toward the schwertmannite and iron oxides stability phases due to changes in pH/redox conditions. The methods presented in this work showed favourable steps toward environmentally sustainable agriculture and improving the chemical and ecological status of acid sulfate soil affected coastal waters.
Photo: Graphic from the publication.