Effects of mitigation suspensions of fine-grained calcite and peat
The publication "Iron‑sulfur geochemistry and acidity retention in hydrologically active macropores of boreal acid sulfate soils: Effects of mitigation suspensions of fine-grained calcite and peat" has been published in Science of The Total Environment, Volume 856, Part 2, 15 January 2023, 159142.
Abstract
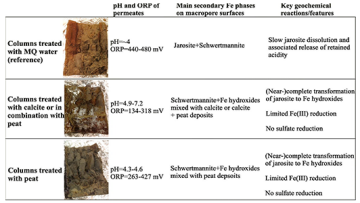
Acid sulfate soils discharge large amounts of sulfuric acid along with toxic metals, deteriorating water quality and ecosystem health of recipient waterbodies. There is thus an urgent need to develop cost-effective and sustainable measures to mitigate the negative effects of these soils. In this study, we flushed aseptically-prepared MQ water (reference) or mitigation suspensions containing calcite, peat or a combination of both through 15-cm-thick soil cores from an acid sulfate soil field in western Finland, and investigated the geochemistry of Fe and S on the surfaces of macropores and in the solid columnar blocks (interiors) of the soil columns. The macropore surfaces of all soil columns were strongly enriched in total and HCl-extractable Fe and S relative to the interiors, owing to the existence of abundant Fe oxyhydroxysulfates (schwertmannite and partly jarosite) as yellow-to-brownish surface-coatings. The dissolution/hydrolysis of Fe oxyhydroxysulfates (predominantly jarosite) on the macropore surfaces of the reference columns, although being constantly flushed, effectively buffered the permeates at pH close to 4. These results suggest that Fe oxyhydroxysulfates accumulated on the macropore surfaces of boreal acid sulfate soils can act as long-lasting acidification sources. The treatments with mitigation suspensions led to a (near-)complete conversion of jarosite to Fe hydroxides, causing a substantial loss of S. In contrast, we did not observe any recognizable evidence indicating transformation of schwertmannite. However, sulfate sorbed by this mineral might be partially lost through anion-exchange processes during the treatments with calcite. No Fe sulfides were found in the peat-treated columns. Since Fe sulfides can support renewed acidification events, the moderate mineralogical changes induced by peat are desirable. In addition, peat materials can act as toxic-metal scavengers. Thus, the peat materials used here, which is relatively cheap in the boreal zone, is ideal for remediating boreal acid sulfate soils and other similar jarosite-bearing soils.
Photo: Graphical abstract