Dredging and deposition of metal sulfide rich river sediments results in rapid conversion to...
The article "Dredging and deposition of metal sulfide rich river sediments results in rapid conversion to acid sulfate soil materials" has been published in Science of The Total Environment, Volume 813, 20 March 2022.
DOI: doi.org/10.1016/j.scitotenv.2021.151864
Abstract
Sediments along the Baltic Sea coast can contain considerable amounts of metal sulfides that if dredged and the spoils deposited such that they are exposed to air, can release high concentrations of acid and toxic metals into recipient water bodies. Two river estuaries in western Finland were dredged from 2013 to 2018 and the dredge spoils were deposited on land previously covered with agricultural limestone to buffer the pH and mitigate acid and metal release. In this study, the geochemistry and 16S rRNA gene amplicon based bacterial communities were investigated over time to explore whether the application of lime prevented a conversion of the dredge spoils into acid producing and metal releasing soil. The pH of the dredge spoils decreased with time indicating metal sulfide oxidation and resulted in elevated sulfate concentrations along with a concomitant release of metals. However, calculations indicated only approximately 5% of the added lime had been dissolved. The bacterial communities decreased in diversity with the lowering of the pH as taxa most similar to extremely acidophilic sulfur, and in some cases iron, oxidizing Acidithiobacillus species became the dominant characterized genus in the deposited dredge spoils as the oxidation front moved deeper. In addition, other taxa characterized as involved in oxidation of iron or sulfur were identified including Gallionella, Sulfuricurvum, and Sulfurimonas. These data suggest there was a rapid conversion of the dredge spoils to severely acidic soil similar to actual acid sulfate soil and that the lime placed on the land prior to deposition of the spoils, and later ploughed into the dry dredge spoils, was insufficient to halt this process. Hence, future dredging and deposition of dredge spoils containing metal sulfides should not only take into account the amount of lime used for buffering but also its grain size and mixing into the soil.
Highlights
- Baltic Sea coastal sediments are rich in metal sulfides.
- Dredging of sediments exposes metal sulfides to oxygen.
- Oxidation of metal sulfide rich estuary sediments releases metals and acid.
- Metal sulfide oxidation coincides with increase of extremely acidophilic bacteria.
- Liming poorly neutralized acid generated from oxidized dredge sediments.
Picture:
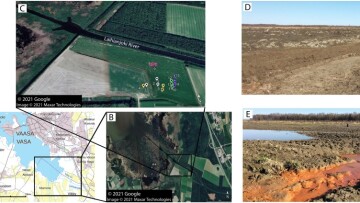
Fig. 1. Map of the dredge spoil study site located south of Laihianjoki River estuary (Sulvanjoki river not shown) near Vaasa, Western Finland (A) with the lower orthophoto showing a zoomed in section of the map (B) and the upper orthophoto shows the dredge spoil sampling sites (C). Sites 1–3 (white dots) were sampled in November 2016, sites 4–6 (yellow dots) in May 2017, sites 7–9 (pink dots) in August 2017, sites 10–12 (green dots) in October 2017, sites 13–15 (purple dots) in May 2018, and reference sediment samples (S1-S3, red dots) in April 2018. The two photographs (both credits: E. Högfors-Rönnholm) show the sampling site in May 2017 (D) and May 2018 (E). The figure contains data from the National Land Survey of Finland Topographic Database (06/2021) and NLS orthophotos (03/2019). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)